Answer the question
In order to leave comments, you need to log in
Why do molecules heat up with the forces of the pendant?
Hello, I have a question more in physics than in programming.
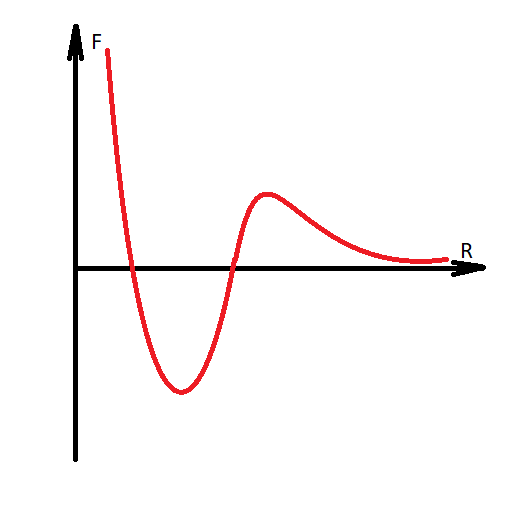
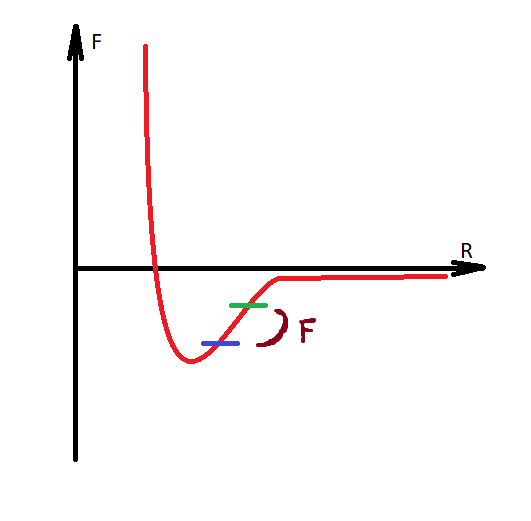
If we consider molecules in a closed space, then 3 main forces will act on them:
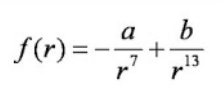
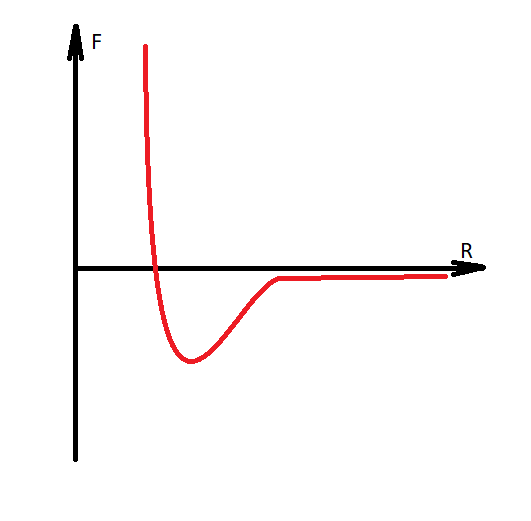
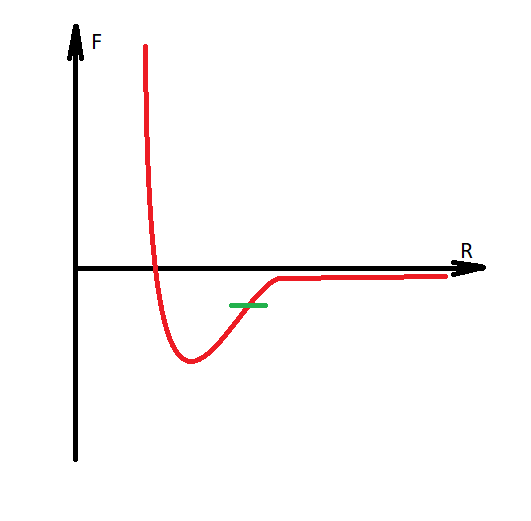
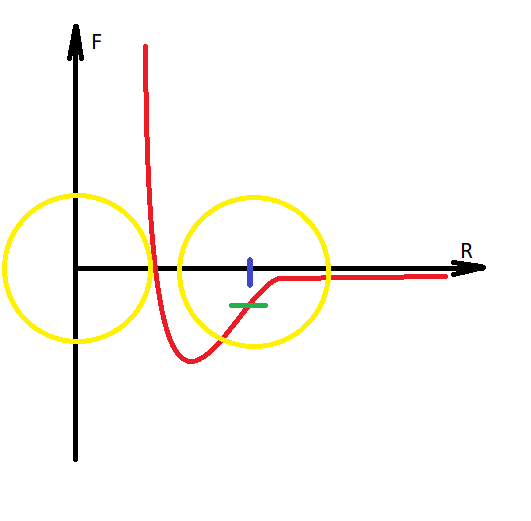
It is necessary to make a digression, that water is modeled with the transition of hydrogen to another molecule, and thus the Coulomb forces are formed between them, since the molecules acquire charges of different signs. Water becomes H30 AND HO.
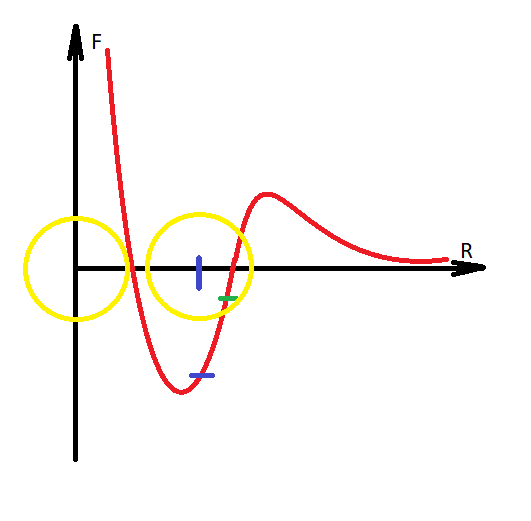
Answer the question
In order to leave comments, you need to log in
How to make sure that the system does not heat up? What am I not considering or could this be a normal situation?You do not take into account that immediately after the transition of the hydrogen nucleus to another molecule, the Coulomb force already begins to act, it reduces the speed of the expansion of molecules, and a decrease in the speed of molecules is cooling. Everything, according to the law of conservation of energy, did not decrease or increase anything, only the energy passed into another form, and then returned to its previous form.
Didn't find what you were looking for?
Ask your questionAsk a Question
731 491 924 answers to any question